Live-attenuated chikungunya vaccine in children: a randomized phase 2 trial
The afternoon sun beat down on the colorful, bustling streets of Santo Domingo, casting long shadows from the swaying palm trees. A young girl, maybe seven years old, chased a stray dog down a cobbled alley, her laughter bright and carefree. Her mother, watching from a doorway, smiled, but a faint crease of worry lingered at the corner of her eyes. It’s a scene of vibrant life, yet beneath the surface, an invisible threat often lurks – the silent hum of a mosquito, carrying with it the potential for a debilitating illness. For families in tropical and subtropical regions across the globe, the fear of chikungunya, with its sudden onset of severe fever and joint pain, is a grim reality that can disrupt lives and livelihoods, leaving a lasting imprint of suffering, particularly on the most vulnerable: children.
Imagine a world where that worry could be significantly lessened, where the playful energy of a child isn’t just a fleeting moment between bouts of sickness, but a sustained state of health, protected by the ingenuity of science. This isn’t merely a hopeful dream; it represents the persistent, often painstaking, work of researchers and medical professionals striving to build defenses against diseases that have long plagued humanity. The journey from understanding a pathogen to developing a safe and effective vaccine is an odyssey marked by rigorous testing, ethical considerations, and an unwavering commitment to public health. It is a journey that, for chikungunya, has recently taken a significant leap forward, offering a beacon of hope for communities where the disease remains a constant specter.
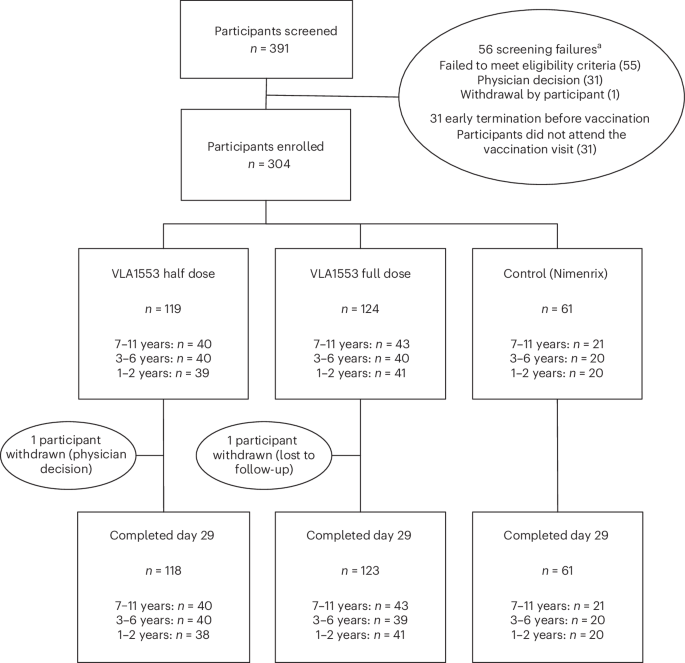
At the heart of this unfolding story is VLA1553, a live-attenuated chikungunya vaccine, which has recently undergone a crucial phase 2 randomized, controlled, dose-response trial. Conducted with meticulous care in the very regions most affected by the virus – Honduras and the Dominican Republic – this study focused specifically on children under the age of 12. The term “live-attenuated” signifies a vaccine that uses a weakened, non-disease-causing form of the virus. This approach allows the body to mount a robust immune response, mimicking a natural infection without the full-blown illness, thereby building lasting protection. The trial meticulously assessed the vaccine’s performance at both full and half doses, scrutinizing its safety profile and its ability to provoke a strong immune reaction, known as immunogenicity. The findings were profoundly encouraging: VLA1553 demonstrated both safety and a potent immune response in this young demographic, marking a pivotal step towards a comprehensive shield against the virus for a population desperately in need of protection.
The significance of these results for a pediatric population cannot be overstated. Developing vaccines for children presents a unique set of challenges, demanding an even higher standard of safety and efficacy. Children’s immune systems are still developing, and their physiological responses can differ from adults. Ensuring that a vaccine is not only effective but also exceptionally well-tolerated in young bodies is paramount. The successful outcomes from this phase 2 trial provide robust data supporting the vaccine’s potential for widespread use in children, paving the way for larger, phase 3 trials that will further confirm these promising initial observations. This methodical progression through clinical trial phases underscores the scientific community’s commitment to ensuring that any medical intervention introduced to the public is thoroughly vetted and meets the highest possible standards for safety and effectiveness.
Chikungunya is not merely an inconvenience; it is a significant public health burden that extends far beyond the initial fever and rash. The name itself, derived from a Makonde word meaning “that which bends up,” vividly describes the chronic, debilitating joint pain that can persist for months, or even years, after the acute infection has passed. This long-term suffering impacts individuals’ ability to work, attend school, and participate fully in daily life, placing immense strain on healthcare systems and economies in endemic regions. The virus is transmitted primarily by Aedes aegypti and Aedes albopictus mosquitoes, which thrive in urban and semi-urban environments, bringing the threat directly into homes and communities. Outbreaks can be explosive, overwhelming local medical facilities and leading to widespread societal disruption.
The global footprint of chikungunya has expanded dramatically in recent decades, moving beyond its traditional range in Africa and Asia to cause major epidemics in Europe, the Americas, and the Caribbean. This geographical spread is fueled by factors such as climate change, increased global travel, and urbanization, which create ideal conditions for mosquito proliferation and virus transmission. In the absence of a specific antiviral treatment, prevention through mosquito control and, critically, vaccination, remains the most effective strategy to mitigate the disease’s impact. The development of VLA1553, particularly its demonstrated efficacy and safety in children, represents a crucial tool in the arsenal against this re-emerging threat. It offers the prospect of reducing illness, preventing long-term disability, and ultimately alleviating the immense pressure on public health infrastructure in vulnerable communities.
For those of us at ‘Wandering Science,’ who bridge the divide between academic rigor and the spirit of exploration, the implications of such scientific advancements resonate deeply. While a non-scientist might not directly witness the intricacies of a randomized controlled trial in a Honduran clinic, they can certainly observe the profound impact of chikungunya in regions where it holds sway. Consider a journey through the vibrant markets of Cartagena, Colombia, or the lush, green landscapes of rural Thailand. These are places where the threat of mosquito-borne diseases is a daily reality, where conversations about fevers and joint pain are common, and where the economic consequences of illness are keenly felt. The local clinics, often stretched thin, are testament to the ongoing battle against such pathogens.
To truly grasp the importance of a vaccine like VLA1553, one might engage with communities in places like the Dominican Republic, seeing firsthand the resilience of families and the hope they hold for a healthier future. Observe the children at play, the elderly struggling with persistent pain, and the public health workers tirelessly educating residents on mosquito prevention. These human stories underscore the vital role of global health initiatives and the tireless dedication of researchers. While we may not all be scientists, we are all global citizens, connected by the shared challenges and triumphs of humanity. Understanding the journey of a vaccine, from lab bench to clinical trial, is not just about appreciating scientific progress; it is about recognizing the profound human impact of exploration and discovery, particularly when it promises to safeguard the most innocent among us.
About admin
A curious explorer documenting the intersection of science and travel. Join the journey to discover the hidden stories of our planet.
Leave a Reply