Chemical capture of diazo metabolites reveals biosynthetic hydrazone oxidation
Imagine a vast, dynamic landscape, teeming with life, yet much of it remains invisible, fleeting, and profoundly mysterious. This isn’t some distant exoplanet, but the microscopic world within our own bodies, in the soil beneath our feet, and in the very air we breathe. Within this unseen realm, microorganisms engage in an intricate chemical ballet, producing a dazzling array of molecules, many of which are so reactive, so ephemeral, that they vanish before traditional scientific methods can even register their presence. For decades, these elusive compounds have been the ‘dark matter’ of natural product chemistry, hinting at biological functions and therapeutic potentials we could only guess at. The challenge has always been how to catch them in the act.
This quest for the chemically invisible has led to a groundbreaking methodological leap, reported in the pages of Nature. Scientists have devised a sophisticated “capture” strategy, a molecular net designed to snag these fleeting chemical entities, specifically focusing on a class of highly reactive compounds known as diazo metabolites. These molecules, characterized by a unique nitrogen-nitrogen double bond, are notorious for their instability, often decomposing rapidly in biological systems. Their very reactivity, however, is also what makes them fascinating; it’s this inherent chemical energy that often underlies their potent biological activities, from antibacterial to anti-cancer effects. The research team’s ingenuity lies in turning this instability into an advantage, creating a system that not only detects these compounds but also stabilizes them for identification.
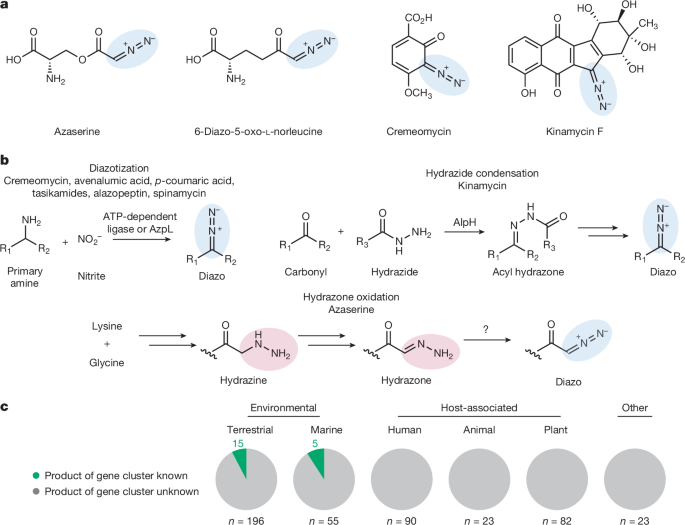
The core of this scientific advancement involves a two-pronged approach: a bespoke chemical capture reagent paired with the precision of mass spectrometry. Think of it as a specialized fishing lure designed to specifically attract and bind to the diazo group, effectively ‘freezing’ the molecule in a stable form. Once captured, these stabilized complexes can then be analyzed by mass spectrometry, a technique that weighs individual molecules, allowing scientists to deduce their exact chemical composition and structure. Applying this innovative methodology, the researchers turned their attention to *Nocardia ninae*, a bacterium known to be an opportunistic human lung pathogen, a member of the diverse *Nocardia* genus often found in soil. What they found was nothing short of a revelation: two previously unknown diazo-containing compounds. More significantly, the study didn’t just identify these new metabolites; it peeled back the layers of their genesis, revealing a novel biosynthetic pathway involving hydrazone oxidation. This discovery challenges prior assumptions about how diazo compounds are formed in nature. Previously, it was thought that these molecules primarily arose from the oxidation of primary amines. The identification of hydrazone oxidation as a distinct and active biosynthetic route fundamentally expands our understanding of microbial metabolism and the intricate enzymatic machinery that crafts these reactive chemicals. This new pathway suggests that the microbial world possesses an even richer toolkit for chemical synthesis than previously appreciated, opening up entirely new avenues for exploring natural product biosynthesis.
The implications of this work stretch far beyond the confines of a microbiology lab. The discovery of new diazo metabolites, especially from a pathogen like *Nocardia ninae*, ignites a spark of excitement in the field of drug discovery. Natural products, particularly those from microorganisms, have historically been a treasure trove for medicinal chemistry, yielding a vast number of antibiotics, anti-cancer agents, and immunosuppressants. Many existing drugs either contain or are inspired by the reactive structures found in nature. The very reactivity that makes diazo compounds difficult to study also endows them with powerful biological activity, often through mechanisms that target essential cellular processes. Understanding the unique biosynthetic pathways that *Nocardia ninae* employs to create these molecules could provide crucial blueprints for synthetic chemists, allowing them to design novel compounds with tailored therapeutic properties. In an era increasingly challenged by antibiotic resistance, the ability to uncover entirely new classes of microbial metabolites, and to understand their origins, offers a renewed hope for developing much-needed new treatments. Furthermore, this methodological breakthrough isn’t limited to *Nocardia ninae* or even to diazo compounds alone. The principle of designing specific capture reagents for reactive, transient molecules holds immense promise for exploring other ‘dark matter’ regions of the metabolome—the complete set of metabolites within a biological organism. This approach could unlock a deeper understanding of fundamental biological processes, reveal hidden signaling molecules, or shed light on how organisms adapt to their environments, all by simply being able to ‘see’ what was previously invisible. It’s a powerful reminder that the exploration of the known world is far from over, merely shifting from vast geographical expanses to the intricate landscapes of molecular biology.
For those of us who yearn to connect with the scientific frontier, to witness the spirit of discovery firsthand, the microscopic world of diazo metabolites may seem too abstract, too far removed from our everyday experience. Yet, the essence of this research—the patient observation, the clever design, the thrill of uncovering hidden truths—can be appreciated in many forms. While you won’t observe diazo compounds with the naked eye, you can connect with the environments where such profound chemical innovation takes place. Imagine strolling through a dense forest, feeling the rich, damp earth beneath your feet. This soil is a bustling metropolis of microbial life, a literal chemical factory where countless bacteria like *Nocardia ninae* are constantly producing, reacting, and transforming molecules, many of which remain undiscovered. Visiting botanical gardens or national parks, particularly those with diverse ecosystems, offers a tangible link to the raw, untamed biodiversity that serves as science’s endless inspiration. Here, the unseen world beneath the leaves and within the soil is a constant reminder of nature’s ingenious chemistry. For a more direct appreciation of the scientific journey, consider visiting a major university research institution or a natural history museum. Many such places offer public exhibitions that showcase the history of microbiology, the tools of modern molecular biology, or the impact of natural product discovery on medicine. The Pasteur Museum in Paris, for instance, offers a captivating glimpse into the foundational work of Louis Pasteur, whose pioneering studies in microbiology laid the groundwork for understanding the very bacteria that continue to surprise us with their chemical prowess. These locations, while not offering a direct view of a diazo metabolite, provide a profound context: they are places where curiosity is cultivated, where the unseen is made visible through human ingenuity, and where the relentless pursuit of knowledge continues to reshape our understanding of life itself. It’s in these spaces, both wild and cultivated, that the wandering scientist—and the wandering enthusiast—can truly feel the pulse of discovery.
About admin
A curious explorer documenting the intersection of science and travel. Join the journey to discover the hidden stories of our planet.
Leave a Reply